| |
| |
Sampling device |
Advantages |
Disadvantages |
|
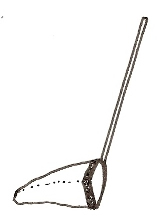
Aquatic D-frame dipnet |
Can sample a variety of habitats within a single wetland using this method (substrate, water column, submerged and emergent vegetation), thus getting an invertebrate sample that represents the diversity of wetland habitats. This method is relatively quick. The same nets used for stream macroinvertebrate collection can be used in wetlands. |
Not a quantitative sampling method; may under-represent certain taxa (i.e. active swimmers, those that reside in the benthos). |
 |
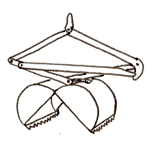
Activity trap/
Funnel trap |
Collects taxa that are fast swimmers and/or those active only at night. Activity traps also will capture amphibians. They can be made cheaply with recycled two liter soda bottles or window screening. |
Captured predators may eat some of the other invertebrates in the traps, resulting in a less representative sample |
|
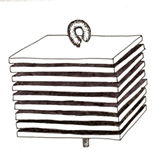
Artificial substrate (i.e. Hester dendy) |
Sampling and sample processing are simpler, less time and labor intensive.. |
Captures only the organisms that colonize this type of substrate, potentially excluding many types of invertebrates. |
|
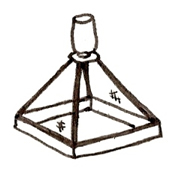
Emergence Traps |
Sorting and identifying the invertebrates is relatively simple. |
Not representative of all wetland taxa because not all invertebrates emerge from the wetland as winged adults (i.e. mollusca, crustacea); traps must be left at a site for a long period of time and may be damaged or lost. |
|
Grab sampler |
Quantitative sampling method that collects invertebrates from the vegetation, water column and wetland substrate. |
Separating invertebrates from abundant organic matter also taken up by the grab is very time consuming. |
|
Stovepipe (or bucket) sampler |
Semiquantitative sampling method that samples invertebrates within the water column and on vegetation. This sampler can be made cheaply by cutting the bottom out of a 5 gallon plastic bucket. |
Time consuming to pick samples because of the large amount of debris and vegetation collected with the samples. |
|
Benthic corer |
Quantitative sampling method for invertebrates living in the benthos, or sediment. |
Time consuming to process samples; not representative of all wetland taxa, only samples those organisms living in the benthos. |
Choosing study sites
The questions you are trying to answer about your watershed will influence which wetlands, and how many, you study. If you are comparing the invertebrates in highly degraded wetlands to those in pristine, or reference, wetlands, it is important to choose your sites carefully. You want the wetlands to be as similar to each other in every way possible (size, subclass, ecoregion, hydrology, geology), except for the level of human impairment. This is to ensure that if you find differences in the invertebrate communities in highly degraded wetlands relative to the invertebrate communities in the reference wetlands, you can be more confident that those differences are due to human impairment, rather than to a natural factor.
If you are doing a study that requires you to choose 'reference' wetland sites, it will be important to consult with regional wetlands experts, and work to find sites that have the least amount of human impact (i.e. the oldest forests, least amount of development or pavement in the surrounding landscape, fewest cattle, etc.).
Which part of a wetland should be sampled
Generally the highest abundance and diversity of invertebrates is found in the parts of a wetland that have emergent vegetation. Different zones within a wetland will probably support different communities of invertebrates, so you may want to sample several different areas to get a representative community of invertebrates. However, if you are sampling multiple wetlands, make sure that you have a standardized method (i.e., always sampling invertebrates in the emergent vegetation zone where the water is 0.5 meters deep). This will allow you to compare the invertebrate community found at Wetland A to the invertebrate community found at Wetland B.
Number of samples to take
There are a few things to consider in making this determination. Ideally, you want to take the number of samples that allows you to capture as much of the actual taxa living in the wetland as possible. If you only take one or two samples, you may miss types of organisms with a patchy distribution. For example, there may be 1,000 planorbid snails colonizing one 3 meter by 3 meter area of the wetland, and none anywhere else. If you take only a couple of samples, and fail to take any samples in this area, you may falsely conclude that there are no planorbid snails living in your wetland.
However, you have to be realistic about the time involved in taking each sample, and more importantly, the time or money it will cost to sort and identify the taxa in each sample. Typically, it will cost from $200-$300 for a professional taxonomist to sort and identify 300 organisms from a sample. One way to maximize the area of a wetland sampled, while still having a reasonable number of samples to process and identify, is to take composite samples, then subsample the organisms in your composite sample for identification. For example, you may take a composite sample of 10 separate sweeps of your dip net through the water, at 10 different locations in the wetland. You would then combine all of the material (vegetation, invertebrates, detritus) from those 10 sweeps into a single 'sample', which may consist of a few jars. There may be a total of 2,356 invertebrates captured in that one sample; obviously, it would be impractical to sort and identify every organism in the sample. Instead, 300-500 macroinvertebrates will be randomly subsampled from that composite sample. In a study conducted by the Xerces Society, three composite samples were taken in each wetland studied, and a subsample of 300 organisms from each sample wa sidentified. When using subsampling, the entire sample is ususally picked for "large and rare" specimens that may not have been represented in the subsample, such as a signle crayfish present in a composite sample.
One way to standardize your sampling is to create a transect, or line, through the wetland, then sample at a specified location on the transect. For example, if you put a 12 meter transect through the wetland, you might sample at 3 meters, 6 meters, 9 meters, and 12 meters. You should do this the same way at each wetland you visit.
Time of year to sample
There are many factors to consider when establishing the ideal sampling index period for a study of wetland invertebrates. Early in the season, many invertebrates will be too small and immature to be able to identify beyond family. As the year progresses, however, wetlands tend to dry out, become choked by vegetation, or colonized by invertebrates from other water bodies. In the Willamette Valley of Oregon, we think that the best sampling period is likely in late May, although we are still refining this optimal index period. In other parts of Oregon or the Pacific Northwest, the optimal sampling index period may differ.
Identifying your samples
You will have to choose between identifying the collected macroinvertebrates yourself or hiring someone to identify them. Identifying invertebrates to species can take a considerable amount of time and expertise. Below is a link to some laboratories in the West that commonly identify samples of stream invertebrates; some of these laboratories also have experience with wetland invertebrate identification.
Laboratories that specialize in invertebrate identification
|